

When Phosphorus Goes Missing in Action - Understanding the Hidden Nutrient Trap
You’ve added the phosphorus.
You’ve followed the recommended rates.
But your crops are still struggling.
What gives?
The answer lies beneath your feet—in the chemistry of your soil.
The Phosphorus Illusion: More Isn’t Always Better
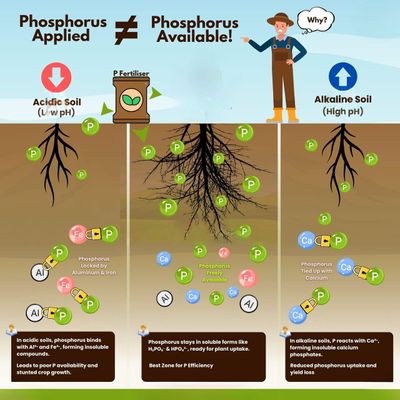
Many growers assume that applying more phosphorus guarantees healthy plant growth. But in reality, phosphorus applied does not automatically mean phosphorus is available. And availability is everything.
Soil isn’t just a storage bin for nutrients—it’s a dynamic, reactive system.
Depending on your soil’s pH, the phosphorus you apply can either help your crops thrive or quietly become useless.
Acidic Soils: Where Phosphorus Gets Trapped by Iron and Aluminum
When soil pH drops below 6, things start to go sideways.
Phosphorus molecules react with iron (Fe³⁺) and aluminum (Al³⁺)—common elements in acidic soils.
The result?
Insoluble compounds that plant roots can’t access.
So even if phosphorus is present in the soil, it's essentially locked up—a buried investment that brings no return.
Alkaline Soils: Calcium Compounds Hijack Your Phosphorus
High pH isn’t innocent either. In alkaline conditions (typically above pH 7.5), phosphorus gets bound by calcium (Ca²⁺), forming calcium phosphate—another insoluble compound.
This chemical reaction ties up phosphorus before plants ever get a chance to absorb it.
Nutrient lockout leads to poor root development, weak shoots, and lower yields.
The Goldilocks Zone: Where Phosphorus Shines
There’s a sweet spot where phosphorus stays soluble, mobile, and plant-accessible—usually between pH 6.0 and 7.0.
In this range, phosphorus remains in usable forms like H₂PO₄⁻ and HPO₄²⁻, easily absorbed by plant roots.
This is where your fertilizer investment actually pays off.
Stop Guessing. Start Testing.
You wouldn’t drive without a fuel gauge—so don’t farm without a soil test.
A simple soil analysis reveals your pH levels and helps you make smart adjustments:
- Raise pH with lime in acidic soils
- Lower pH with elemental sulfur or acid-forming fertilizers in alkaline zones
- Choose the right type of phosphorus fertilizer for your specific soil conditions
Don't Let Your Fertilizer Go to Waste
When phosphorus gets locked up, it’s not just a lost nutrient—it’s a lost opportunity:
💸 Wasted fertilizer
📉 Reduced yields
🌱 Poor plant health
And the worst part? You may never realize the problem isn’t the phosphorus itself—it’s the soil’s reaction to it.
The Takeaway: It's Not What You Add. It's What Plants Can Use.
Feed the soil wisely.
Balance the pH.
Unlock the full power of your phosphorus.
Because the most expensive fertilizer…
is the one your plants can’t use.
