

- Home
- Companies
- MSD Animal Health
- Products
- Cobactan 4.5%
Cobactan 4.5%
Cobactan 4.5% is a powder and solvent for solution for injection. It is for the treatment of bacterial infections in horses and cattle caused by the following Gram positive and Gram negative microorganisms sensitive to cefquinome. Cobactan 4.5% can may only be prescribed by your veterinary surgeon from whom advice should be sought. Further information is available on request.
Presentation
Powder and solvent for solution for injection.
1 vial with powder contains:
30 ml vial: 1.35 g cefquinome (as sulphate)
100 ml vial: 4.5 g cefquinome (as sulphate)
1 ml solvent contains:
Preservative: Benzyl alcohol 10 mg (E1519)
Each ml of the reconstituted solution contains:
Active substance:
Cefquinome (as sulphate) 45 mg
Excipient:
Benzyl alcohol (E1519) 10 mg
Uses
For the treatment of bacterial infections in horses and cattle caused by the following Gram positive and Gram negative microorganisms
sensitive to cefquinome:
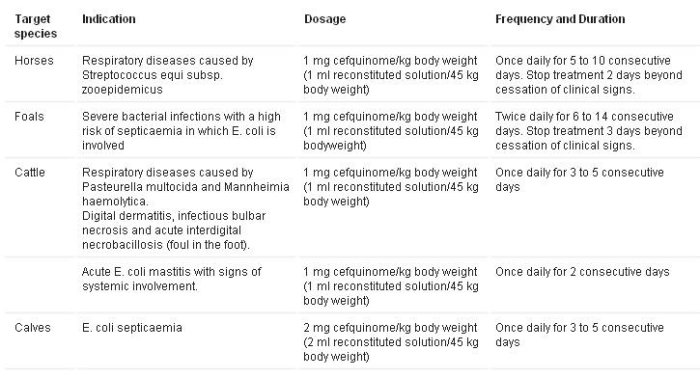
Horses:
- Respiratory diseases caused by Streptococcus equi subsp. zooepidemicus.
Foals:
- Severe bacterial infections with a high risk of septicaemia in which Escherichia coli is involved.
Cattle:
- Respiratory diseases caused by Pasteurella multocida and Mannheimia haemolytica.
- Digital dermatitis, infectious bulbar necrosis and acute interdigital necrobacillosis (foul in the foot).
- Acute E. coli mastitis with signs of systemic involvement.
Calves:
- E. coli septicaemia.
Dosage and administration
Reconstitute the solution for injection by adding the whole contents of the solvent vial. After reconstitution, shake the vial well before
use. Do not use another solvent (e.g. water for injection). Swab the rubber stopper before removing each dose. Use a dry sterile needle
and syringe. An appropriately graduated syringe must be used to allow accurate administration of the required dose volume. The rubber
stopper may be punctured up to 20 times.
See Dosage table: Cobactan 4.5%
Cattle and calves: by intramuscular injection, and restricted to 10 ml per injection site.
It is recommended to administer the product into different injection sites.
In cattle the preferred injection site is in the muscular tissue of the mid-neck region.
Horses and foals: injections can be given either intramuscularly or intravenously.
In foals, it is recommended to start the treatment with intravenous injections for 3 days and to follow with intramuscular injections.
To ensure the correct dosage the body weight should be determined as accurately as possible to avoid possible underdosing.
Contra-indications, warnings, etc
Do not use in animals which are known to be hypersensitive to cephalosporin antibiotics, other ß-lactam antibiotics or to any of the excipients.
Use of the product should be based on susceptibility testing of the bacteria isolated from the animal. If this is not possible, therapy should be based on local (regional, farm level) epidemiological information about susceptibility of the target bacteria. Use of the product
deviating from the instructions given in the SPC or the package leaflet may increase the prevalence of bacteria resistant to cefquinome and may decrease the effectiveness of treatment with cephalosporins, due to the potential for cross-resistance.
Hypersensitivity reactions to cephalosporins occur rarely. In horses, occasionally slight transient reactions may occur at the injection site. In cattle, use of the product may result in localized tissue reaction. Tissue lesions are resolved 15 days after the last injection.
There is no information on reproductive toxicity (incl. teratogenicity) in horses and cattle. Laboratory studies in rats and rabbits have not
shown any teratogenic, foetotoxic or maternotoxic effects. Use only according to the benefit/risk assessment by the responsible veterinarian.
Doses of 20 mg/kg in cattle, 1 mg/kg in horses and 3 mg/kg in foals twice daily have been well tolerated.
Operator warnings
Penicillins and cephalosporins may cause hypersensitivity (allergy) following injection, inhalation, ingestion or skin contact.
Hypersensitivity to penicillins may lead to cross sensitivity to cephalosporins and vice versa. Allergic reactions to these substances may occasionally be serious.
- Do not handle this product if you know you are sensitized, or if you have been advised not to work with such preparations.
- Handle this product with great care to avoid exposure, taking all recommended precautions.
- If you develop symptoms following exposure such as a skin rash, you should seek medical advice and show the doctor this warning.
Swelling of the face, lips or eyes or difficulty with breathing, are more serious symptoms and require urgent medical attention.
In case of accidental contact with eyes, rinse immediately with copious amounts of water. Accidental spillage on the skin should be washed off immediately with soap and water. In case of accidental self-injection, seek medical advice immediately and show the package leaflet or the label to the physician.
Withdrawal periods
Horse
Horse meat: 4 days.
Not to be used in horses producing milk for human consumption.
Cattle
Meat: 2 days.
Milk: 36 hours (3 milkings).
For animal treatment only. Keep out of the reach and sight of children.
Pharmaceutical precautions
The powder and solvent vials do not require any special storage conditions.
Shelflife after dilution or reconstitution according to directions: 10 days when stored in a refrigerator (2°C - 8°C). After this time, product
remaining in the container should be discarded.
Dispose of any unused product and empty containers in accordance with guidance from your local waste regulation authority.
In the absence of compatibility studies, this veterinary medicinal product must not be mixed with other veterinary medicinal products.
Legal category
POM-V
Packaging Quantities
Carton containing one 30 ml powder vial and one 30 ml solvent vial.
Carton containing one 100 ml powder vial and one 100 ml solvent vial. Not all pack sizes may be marketed.
Further information
Nil
Marketing Authorisation Holder (if different from distributor)
Intervet International BV, PO Box 31, 5830 AA Boxmeer, The Netherlands
Marketing authorisation number
Vm 06376/4059.
